Label Development & Printing
Biorep offers a full range of medical device labeling services designed to meet your unique specifications and industry regulations. From the design of your label to ensuring labels meet domestic and international label compliance requirements, Biorep works diligently to deliver precise label printing for all your medical devices.
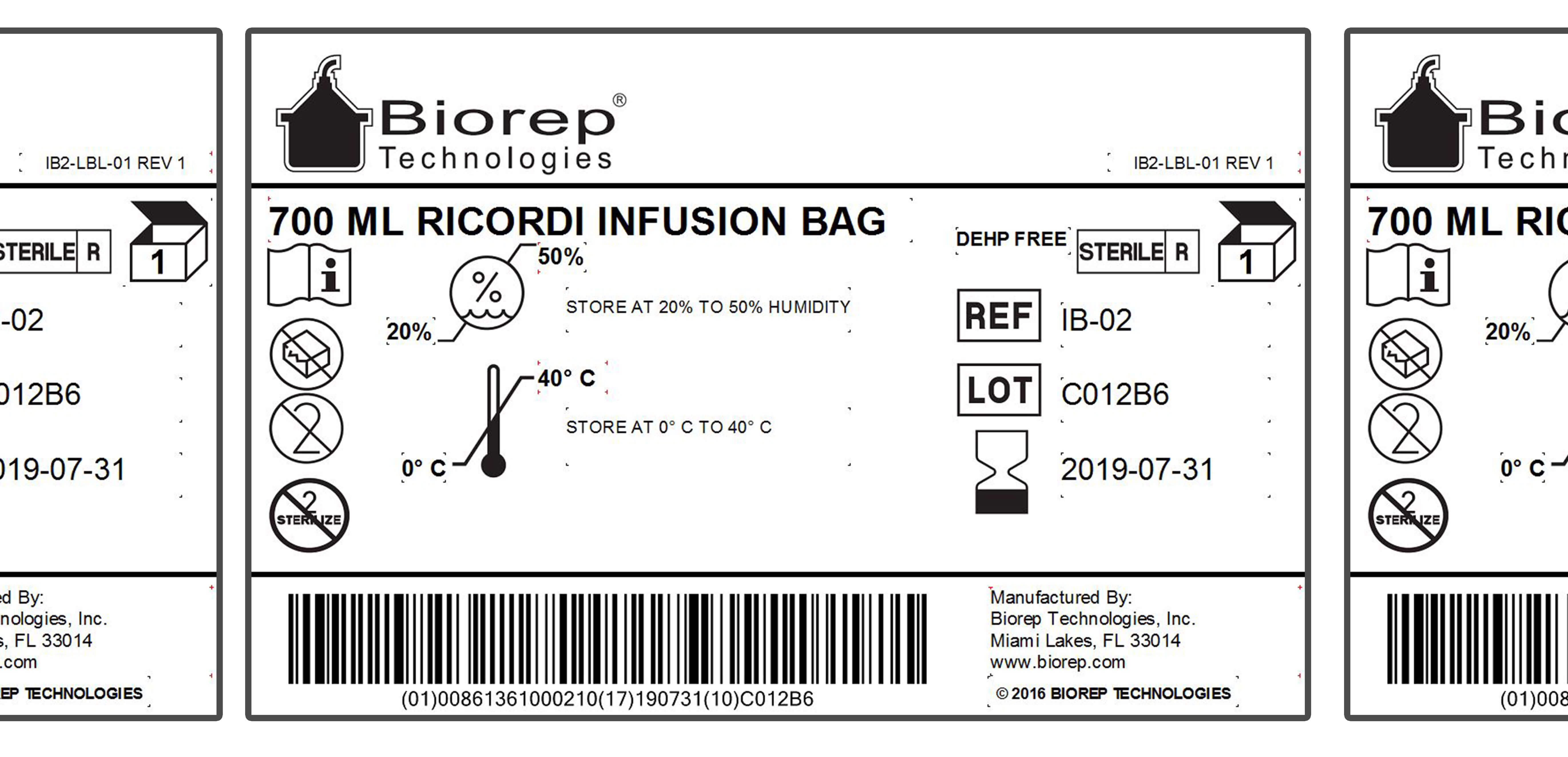
Biorep offers a validated labeling system to ensure compliance with the Code of Federal Regulations (CFR) and ISO label content guidelines. Our labeling system at Biorep brings our clients an enterprise level solution for all your product labeling needs. We can help customers implement GS1 package barcodes, and can assist clients with understanding and implementation of Unique Device Identification (UDI) compliant labeling.

